Know more about
Dissolution Testers
100 % USP/EP/JP compliant
USP methods 1, 2, 4, 5 and 6
Intuitive Touch interface
Dissolution Testers
Product List

Dissolution Tester DS 8000 Basic
The Dissolution Tester DS 8000 Basic by Labindia Analytical is an efficient and user-friendly solution for dissolution testing in pharmaceutical laboratories.
Explore More
Dissolution Tester DS 14000 Basic
The Dissolution Tester DS 14000 Basic by Labindia Analytical offers a reliable and cost-effective solution for dissolution testing.
Explore More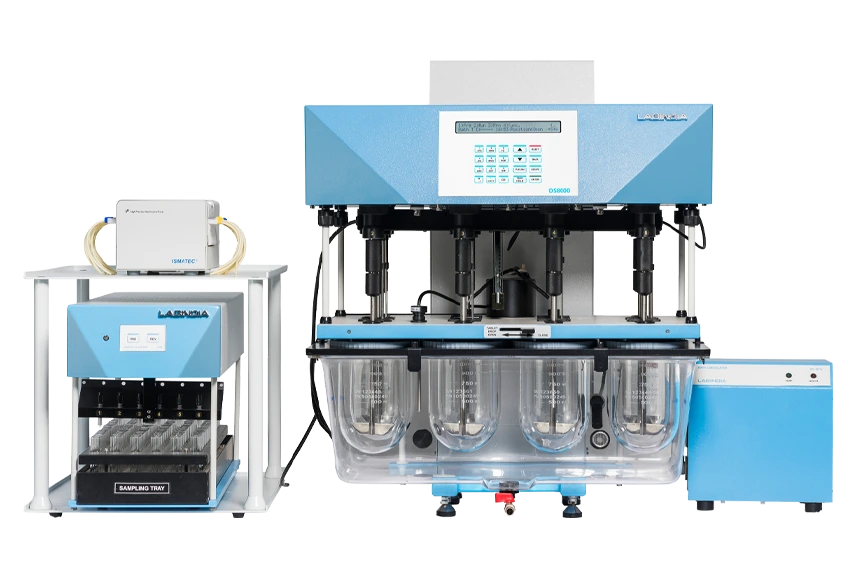
DS 8000 Dissolution Apparatus with Peristaltic Pump
Labindia Analytical’s DS 8000 Dissolution Apparatus with a peristaltic pump ensures consistent fluid delivery for accurate dissolution testing.
Explore More
Tablet Dissolution Tester DS 8000+ with Piston Pump & Automatic Filter Changer
The Labindia Tablet Dissolution Tester DS 8000+ with Piston Pump and Automatic Filter Changer is the ideal solution for streamlined dissolution testing.
Explore More
Tablet Dissolution Tester DS 14000 with Peristaltic Pump
The Tablet Dissolution Tester DS 14000 with a peristaltic pump offers a high-throughput solution for dissolution testing, adhering to the latest USP, EP, BP, and IP standards.
Explore More
Tablet Dissolution Tester DS 8000+ with Syringe Pump
The Labindia Tablet Dissolution Tester DS 8000+ with Syringe Pump is an ideal semi-automated system for routine testing.
Explore More
Tablet Dissolution Tester DS 8000+ with Piston Pump
The Labindia Tablet Dissolution Tester DS 8000+ with Piston Pump offers a semi-automated solution for precise and efficient drug dissolution testing.
Explore More
Tablet Dissolution Tester DS 14000+ with Piston Pump
The Tablet Dissolution Tester DS 14000+ with Piston Pump is designed for high-throughput, accurate dissolution testing.
Explore More
Tablet Dissolution Tester DS 8000 (Basic) SMART
Introducing the Tablet Dissolution Tester DS 8000 (Basic) SMART, an advanced and reliable solution designed to meet the latest pharmaceutical testing standards.
Explore More
Tablet Dissolution Tester DS 14000 (Basic) SMART
Introducing the Tablet Dissolution Tester DS 14000 (Basic) SMART, the ideal solution for accurate and efficient dissolution testing.
Explore More
Tablet Dissolution Tester DS 8000 SMART with Piston Pump
The Labindia Tablet Dissolution Tester DS 8000 SMART with Piston Pump is an advanced semi-automatic solution for precise and efficient drug dissolution testing.
Explore More
Tablet Dissolution Tester DS 14000 SMART with Piston Pump
The Labindia Tablet Dissolution Tester DS 14000 SMART with Piston Pump is a cutting-edge system designed for accurate and efficient drug dissolution testing.
Explore More
Dissolution Tester DS 14000+ with Syringe Pump
The Labindia Tablet Dissolution Tester DS 14000+ with Syringe Pump offers advanced capabilities for efficient and accurate offline dissolution sampling.
Explore More
Dissolution Vessel Washer DVW 1
One of the time-consuming and an essential quality control activity after conducting the dissolution testing is the cleaning of the apparatus
Explore More
Dissolution Vessel Washer DVW 2
It is imperative to clean-up the apparatus thoroughly after conducting dissolution testing in order to avoid contamination and carryover.
Explore More
USP Apparatus 4 – Flow-Through Dissolution System
Designed to reveal formulation differences that conventional USP Apparatus 1 and 2 might miss, this system is especially effective for poorly soluble drugs, modified/extended release formulations, and low-dose products. With growing relevance in IVIVC (in vitro–in vivo correlation) studies and non-traditional dosage forms, the USP Apparatus 4 continues to evolve, adapting to the dynamic needs of modern pharmaceutical testing.
Explore More